FDA renews debate over biased pulse oximeters
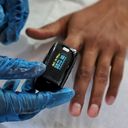
A Food and Drug Administration expert panel on Friday is set to resume the pandemic-driven debate over how to make pulse oximeters more accurate for people with darker skin.
Why it matters: There's growing evidence that the devices don't reliably detect low oxygen levels in Black patients, resulting in delayed care, missed diagnoses of hypoxemia and possibly worse outcomes.
- The COVID-19 crisis revealed how bias in the devices, which led to treatment delays for Black patients with the virus, could exacerbate disparities in the treatment of other conditions like COPD and emphysema.
- After issuing a formal warning about the device's limitations in early 2021 and convening an advisory panel late in 2022, the FDA is now weighing updating manufacturer testing standards it issued more than a decade ago — and seeking experts' input.
- 25 state attorneys general in November prodded the agency to move quickly to improve oximeter accuracy "to prevent additional severe illness and mortalities among darker skinned people" as the nation emerges from the pandemic.
How it works: Pulse oximeters measure the amount of light that passes through a patient's skin to estimate the amount of oxygen in red blood cells.
- The problem is the technology can misjudge oxygen saturation in patients with darker skin. Researchers have suggested updates, including using software algorithms to iron out discrepancies.
Driving the news: With no consensus on the best way to assess skin pigmentation for medical device development, the FDA is proposing updated standards for clinical studies to "promote a more inclusive and representative" patient population.
- It would call for at least 24 participants who span the Monk Skin Tone scale — a 10-shade metric developed with and incorporated into Google products at first to train computer vision models.
- Participants would also have pigmentation measured by an instrument where the pulse oximeter sensor is placed, to improve consistency and offer a better understanding of performance differences.
- The expert panel is being asked to weigh whether the proposal will achieve inclusivity goals, as well as considerations for studies of over-the-counter oximeters for consumer use.
The big picture: Pulse oximeters increasingly have been seen as an important wellness feature in consumer tech and were central to a patent fight between Apple and medical technology company Masimo that resulted in the function being disabled on new Apple Watches.
- Apple also previously faced a class action lawsuit over the accuracy of its watch's pulse oximetry in darker skin tones, but it was dismissed last summer.
- The FDA in August cleared a smart ring from BodiMetrics that the company says can accurately measure blood oxygen in patients with darker skin tones.
What's next: While the agency doesn't have to act on the experts' recommendations, it's facing increasing pressure to fix a high-profile problem that's become emblematic of the racial bias pervasive in medicine.
- "We shouldn't putz around. It's as important in people with respiratory diseases as blood glucose for diabetics," said Meir Kryger, a retired sleep researcher and professor emeritus at the Yale School of Medicine.
- “It's an important measurement, and the doctor or the consumer who's told to get one of these needs to have the confidence that the device is actually accurate."