Another prospect for an Alzheimer's drug renews cost concerns
The prospect of an effective new Alzheimer's treatment came roaring back this week with the announcement of preliminary clinical trial data, giving millions of seniors renewed hope after a tumultuous year.
Why it matters: Alzheimer's is a devastating disease, and the topline results boosted analysts' expectations for an entire class of drugs targeting the condition. But they also resurrect enormous questions about who'll cover the costs and how the U.S. will oversee what's likely a multi-billion dollar market.
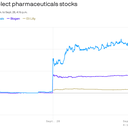
Driving the news: The success of Eisai and Biogen's clinical trial of lecanemab, reported Tuesday night, has instantly changed the narrative around arresting Alzheimer's progression.
- Drugmakers have spent billions of dollars developing products built around the theory that brain amyloid plaques are major contributors to Alzheimer's and that reducing those plaques will fight the disease.
- But the expectations for those drugs were dashed by the drawn-out saga of Biogen's other Alzheimer's drug, Aduhelm, which was approved by the FDA last year without solid evidence it worked.
- Medicare administrators this spring placed coverage limits on any treatments that target amyloid plaques but haven’t shown a clear benefit, meaning Aduhelm was largely excluded from the program.
State of play: The lecanemab news ironically came the same day that the Biden administration announced lower Medicare premiums in 2023, a product of Aduhelm's coverage limitations.
- The trial results — announced by press release — will likely restart the entire regulatory mechanism, this time for a drug that experts say has gone through a much more reliable scientific process.
- "So far, what we know about lecanemab are data that come from what I call normal science, whereas the data we had from [Aduhelm] came from abnormal science, and that’s a big difference," said Jason Karlawish, a medical professor at the University of Pennsylvania.
- Eli Lilly, Roche and Acumen Pharmaceuticals also have drugs in development that target amyloid plaques, and the Biogen results have raised many investors' expectations for their success.
The other side: Some in the research community continue to question the focus on anti-amyloid cures and say success fighting Alzheimer's will come in combination therapies like those used for heart disease, cancer and hypertension.
- "Amyloid-clearing drugs will provide an incremental benefit at best and there is still a pressing need for the next generation of drugs focused on other targets based on our knowledge of the biology of aging," said Howard Fillit, co-founder and chief science officer of the Alzheimer's Drug Discovery Foundation.
Between the lines: Aduhelm's approval created the possibility of a nightmare spending scenario after it was initially priced at $56,000 a year and approved for all Alzheimer's patients.
- Even with significantly lower prices and a narrower eligibility criteria, a new Alzheimer's drug would almost certainly cost the U.S. health system billions every year.
- Eisai has said that lecanemab's "annual value-based price" could be between $9,249 and $35,605.
What they're saying: If the FDA approves lecanemab, the Centers for Medicare and Medicaid Services will once again be in the position of determining if and how to cover it.
- "If the data is what the companies say it is, we believe it will be hard for CMS to refuse coverage," said Raymond James analyst Chris Meekins, who estimates the earliest Medicare could start reimbursing for the drug is late in the third quarter of 2023.
- "FDA approval seems likely, but CMS determination [is] another bridge to cross," Wedbush's Laura Chico told investors Wednesday morning per MarketWatch. "It's a positive to see the study reach statistical significance, with FDA previously signaling this would be a prerequisite for approval. However, what CMS will see as a clinically meaningful outcome remains to be determined."
What we're watching: Full details of the trial will be presented at a scientific conference on Nov. 29. Although the drug is currently on track to undergo accelerated approval by the FDA, the data could justify traditional approval, analysts said.
The bottom line: "If what they report is all proper and true and valid ... and holds up to scrutiny, we have an effective treatment for Alzheimer's disease," Karlawish said.