CDC recommends Pfizer boosters for 12- to 17-year-olds
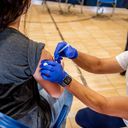
Centers for Disease Control and Prevention director Rochelle Walensky on Wednesday approved a CDC advisory committee's vote to recommend booster doses of Pfizer-BioNTech's COVID-19 vaccine for 12- to 17-year-olds.
Driving the news: The vote passed 13-1 earlier on Wednesday and will expand the number of children eligible for boosters as a surge of COVID-19 cases driven by the Omicron variant spreads nationwide.
- The booster shot should come five months after their initial vaccination series, according to a CDC statement.
- The surge coincides with students' return to school following the holiday break.
What they're saying: "It is critical that we protect our children and teens from COVID-19 infection and the complications of severe disease," Walensky said.
The big picture: The move comes days after the Food and Drug Administration expanded its authorization of Pfizer boosters to allow 12- to 15-year-olds to receive the third shots.
- The CDC on Tuesday also updated its COVID-19 vaccine guidance, recommending that individuals who received the Pfizer shot get a booster five months after getting their second shot, instead of six.
- The CDC also encouraged children who are immunocompromised between the ages of 5 and 11 to receive a third primary COVID-19 shot 28 days after their second shot.
Go deeper: CDC shortens Pfizer booster wait time to 5 months after vaccine
Editor's note: This story has been updated to reflect that the CDC accepted the advisory panel's recommendation.