Tuning reaction barriers for carbon dioxide electroreduction to multicarbon products
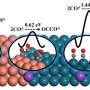
Using intermittent electric energy to convert excessive CO2 into C2 products, such as ethylene and ethanol, is an effective strategy to mitigate the greenhouse effect. Copper (Cu) is the only single metal catalyst which can electrochemically convert CO2 into C2 products, albeit with undesirable selectivity of the C2 product. Therefore, improving the conversion efficiency of Cu-based catalysts for reducing CO2 to C2 products has attracted great attention.